New article in Magnetic Resonance in Chemistry
02 September 2022The assignment of 11B and 1H resonances in the post-reaction mixture from the dry synthesis of Li(BH3NH2BH2NH2BH3)Enhanced Nuclear Magnetic Resonance Spectroscopy with Isotropic Mixing as a Pseudodimension
Ewa K. Nawrocka, Agnieszka Prus, Rafał Owarzany, Wiktor Koźmiński, Krzysztof Kazimierczuk, Karol J. Fijalkowski

We report a detailed 1H NMR and11B NMR study of as synthesised LiBH3NH2BH2NH2BH3Þobtained in a novel dry-synthesis method. A combination of 1D and 2D single- and triple-quantum techniques was used for the assignment of all observed signals. Minor side-products andreactants were detected in the product: NH3BH3,LiNH2BH3 LiBH4,and two yet unknown salts containing 7-membered chain anions:ðBH3NH2BH2NH2BH2NH2BH3 and BHðNH2BH3 We believe the assignment provided within this study might be helpful when analysing the mixtures containing numerous ammonia borane derivatives, which often give overlapping signals that are hard to distinguish
New article in Dalton Transactions
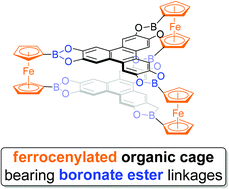
Design of a D3h-symmetry prismatic tris-(ferrocene-1,1′-diyl) molecular cage bearing boronate ester linkages
Maurycy Krzyżanowski, Anna M. Nowicka, Krzysztof Kazimierczuk, Krzysztof Durka, Sergiusz Luliński and Artur Kasprzak
This paper presents a simple, highly selective, and efficient (isolated yield of 68%) synthesis of a novel D3h-symmetry prismatic tris-(ferrocene-1,1′-diyl) organic cage (FcB-cage) by incorporating a boronate ester as a linkage motif. 1,1′-Diboronated derivatives of ferrocene and 2,3,6,7,10,11-hexahydroxytriphenylene (HHTP) were used as the starting materials. The synthesized cage was comprehensively characterized by spectroscopic and microscopic methods, powder X-ray diffraction, thermogravimetry and voltammetry. Cyclic voltammetry analysis revealed the electronic communication between the ferrocene units of the FcB-cage. In addition, to better understand the mechanism behind the synthesis of such a cage, as well as its geometric properties, we performed DFT calculations.
New article in Microporous and Mesoporous Materials
Dipole-dipole interactions of sulfone groups as a tool for self-assembly of a 2D Covalent Organic Framework derived from a non-linear diboronic acid
Krzysztof Durka, Krzysztof Kazimierczuk, Sergiusz Luliński

The utility of the nonlinear diboronic acid 2, obtained from 3,7-dibromodibenzo[b,d]thiophene 5,5-dioxide 1, for the preparation of Covalent Organic Frameworks was investigated. Despite significant deviation of boronic groups in 2 from the colinear arrangement, a highly crystalline porous material DBSO-COF was obtained by dehydrative polycondensation with 2,3,6,7,10,11-hexahydroxytriphenylene (HHTP) using a solvothermal approach. Subsequent PXRD studies supported by periodic DFT modelling revealed the formation of the 2D honeycomb-type lattice with eclipsed stacking model. The antiparallel orientation of neighboured layers is strongly favoured owing to dipole-dipole and C–H⋯O interactions of sulfone moieties. This gives the net stabilization energy of 45 kJ mol−1 per single sulfone motif, being primarily responsible for the effective formation of the ordered network and strongly contributing to its thermodynamic stability. The morphology was analyzed by SEM which revealed that the material forms uniform in size ca. 40 × 150 nm rod-like nano-crystallites. The Grand Canonical Monte Carlo (GCMC) simulation performed on a single nanoparticle of DBSO-COF allowed to reproduce the experimental isotherm. It also showed that N2 molecules are mostly located close to the C–H bonds, while they are repulsed by sulfone groups. Apart from increasing the scope of useful boronic linkers beyond centrosymmetric structures, it was demonstrated that the presence of guest molecules in a porous network should be taken into account in order to obtain a more accurate prediction of porosity parameters.
New Article in Chemistry of Materials
10 May 2022Diazonium-Based Covalent Molecular Wiring of Single-Layer Graphene Leads to Enhanced Unidirectional Photocurrent Generation through the p-doping Effect
Margot Jacquet, Silvio Osella, Ersan Harputlu, Barbara Pałys, Monika Kaczmarek, Ewa K. Nawrocka, Adam A. Rajkiewicz, Marcin Kalek, Paweł P. Michałowski, Bartosz Trzaskowski, C. Gokhan Unlu, Wojciech Lisowski, Marcin Pisarek, Krzysztof Kazimierczuk, Kasim Ocakoglu, Agnieszka Więckowska, and Joanna Kargul

Development of robust and cost-effective smart materials requires rational chemical nanoengineering to provide viable technological solutions for a wide range of applications. Recently, a powerful approach based on the electrografting of diazonium salts has attracted a great deal of attention due to its numerous technological advantages. Several studies on graphene-based materials reveal that the covalent attachment of aryl groups via the above approach could lead to additional beneficial properties of this versatile material. Here, we developed the covalently linked metalorganic wires on two transparent, cheap, and conductive materials: fluorine-doped tin oxide (FTO) and FTO/single-layer graphene (FTO/SLG). The wires are terminated with nitrilotriacetic acid metal complexes, which are universal molecular anchors to immobilize His6-tagged proteins, such as biophotocatalysts and other types of redox-active proteins of great interest in biotechnology, optoelectronics, and artificial photosynthesis. We show for the first time that the covalent grafting of a diazonium salt precursor on two different electron-rich surfaces leads to the formation of the molecular wires that promote p-doping of SLG concomitantly with a significantly enhanced unidirectional cathodic photocurrent up to 1 μA cm–2. Density functional theory modeling reveals that the exceptionally high photocurrent values are due to two distinct mechanisms of electron transfer originating from different orbitals/bands of the diazonium-derived wires depending on the nature of the chelating metal redox center. Importantly, the novel metalorganic interfaces reported here exhibit minimized back electron transfer, which is essential for the maximization of solar conversion efficiency.
New Article in Microporous and Mesoporous Materials
Dipole-dipole interactions of sulfone groups as a tool for self-assembly of a 2D Covalent Organic Framework derived from a non-linear diboronic acid
Krzysztof Durka, Krzysztof Kazimierczuk, Sergiusz Luliński

The utility of the nonlinear diboronic acid 2, obtained from 3,7-dibromodibenzo[b,d]thiophene 5,5-dioxide 1, for the preparation of Covalent Organic Frameworks was investigated. Despite significant deviation of boronic groups in 2 from the colinear arrangement, a highly crystalline porous material DBSO-COF was obtained by dehydrative polycondensation with 2,3,6,7,10,11-hexahydroxytriphenylene (HHTP) using a solvothermal approach. Subsequent PXRD studies supported by periodic DFT modelling revealed the formation of the 2D honeycomb-type lattice with eclipsed stacking model. The antiparallel orientation of neighboured layers is strongly favoured owing to dipole-dipole and C–H⋯O interactions of sulfone moieties. This gives the net stabilization energy of 45 kJ mol−1 per single sulfone motif, being primarily responsible for the effective formation of the ordered network and strongly contributing to its thermodynamic stability. The morphology was analyzed by SEM which revealed that the material forms uniform in size ca. 40 × 150 nm rod-like nano-crystallites. The Grand Canonical Monte Carlo (GCMC) simulation performed on a single nanoparticle of DBSO-COF allowed to reproduce the experimental isotherm. It also showed that N2 molecules are mostly located close to the C–H bonds, while they are repulsed by sulfone groups. Apart from increasing the scope of useful boronic linkers beyond centrosymmetric structures, it was demonstrated that the presence of guest molecules in a porous network should be taken into account in order to obtain a more accurate prediction of porosity parameters.
New Article in Magnetic Resonance
NUScon: a community-driven platform for quantitative evaluation of nonuniform sampling in NMR
Yulia Pustovalova, Frank Delaglio, D. Levi Craft, Haribabu Arthanari, Ad Bax, Martin Billeter, Mark J. Bostock, Hesam Dashti, D. Flemming Hansen, Sven G. Hyberts, Bruce A. Johnson, Krzysztof Kazimierczuk, Hengfa Lu, Mark Maciejewski, Tomas M. Miljenović, Mehdi Mobli, Daniel Nietlispach, Vladislav Orekhov, Robert Powers, Xiaobo Qu, Scott Anthony Robson, David Rovnyak, Gerhard Wagner, Jinfa Ying, Matthew Zambrello, Jeffrey C. Hoch, David L. Donoho, and Adam D. Schuyler
![]()
Although the concepts of nonuniform sampling (NUS) and non-Fourier spectral reconstruction in multidimensional NMR began to emerge 4 decades ago (Bodenhausen and Ernst, 1981; Barna and Laue, 1987), it is only relatively recently that NUS has become more commonplace. Advantages of NUS include the ability to tailor experiments to reduce data collection time and to improve spectral quality, whether through detection of closely spaced peaks (i.e., “resolution”) or peaks of weak intensity (i.e., “sensitivity”). Wider adoption of these methods is the result of improvements in computational performance, a growing abundance and flexibility of software, support from NMR spectrometer vendors, and the increased data sampling demands imposed by higher magnetic fields. However, the identification of best practices still remains a significant and unmet challenge. Unlike the discrete Fourier transform, non-Fourier methods used to reconstruct spectra from NUS data are nonlinear, depend on the complexity and nature of the signals, and lack quantitative or formal theory describing their performance. Seemingly subtle algorithmic differences may lead to significant variabilities in spectral qualities and artifacts. A community-based critical assessment of NUS challenge problems has been initiated, called the “Nonuniform Sampling Contest” (NUScon), with the objective of determining best practices for processing and analyzing NUS experiments. We address this objective by constructing challenges from NMR experiments that we inject with synthetic signals, and we process these challenges using workflows submitted by the community. In the initial rounds of NUScon our aim is to establish objective criteria for evaluating the quality of spectral reconstructions. We present here a software package for performing the quantitative analyses, and we present the results from the first two rounds of NUScon. We discuss the challenges that remain and present a roadmap for continued community-driven development with the ultimate aim of providing best practices in this rapidly evolving field. The NUScon software package and all data from evaluating the challenge problems are hosted on the NMRbox platform.