New Article in Sensors
02 March 2020Enhancing Compression Level for More Efficient Compressed Sensing and Other Lessons from NMR Spectroscopy
Dariusz Gołowicz, Paweł Kasprzak and Krzysztof Kazimierczuk*
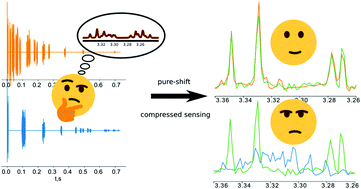
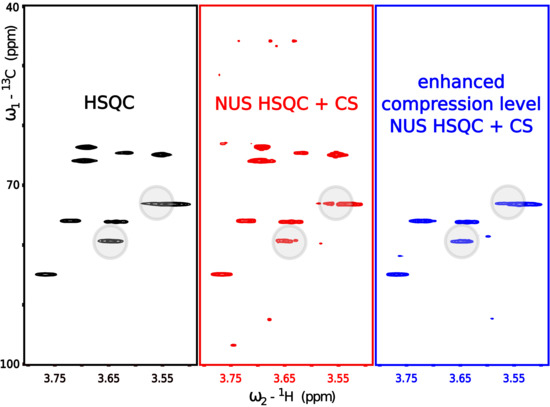
Modern nuclear magnetic resonance spectroscopy (NMR) is based on two- and higher-dimensional experiments that allow the solving of molecular structures, i.e., determine the relative positions of single atoms very precisely. However, rich chemical information comes at the price of long data acquisition times (up to several days). This problem can be alleviated by compressed sensing (CS)—a method that revolutionized many fields of technology. It is known that CS performs the most efficiently when measured objects feature a high level of compressibility, which in the case of NMR signal means that its frequency domain representation (spectrum) has a low number of significant points. However, many NMR spectroscopists are not aware of the fact that various well-known signal acquisition procedures enhance compressibility and thus should be used prior to CS reconstruction. In this study, we discuss such procedures and show to what extent they are complementary to CS approaches. We believe that the survey will be useful not only for NMR spectroscopists but also to inspire the broader signal processing community.
New Article in ChemPhysChem
23 January 2020Blue‐shift hydrogen bonds in silyltriptycene derivatives. Antibonding σ* orbitals of Si‐C bond as effective acceptors of electron density
Tomasz Ratajczyk*, Adam Mames, Dariusz Gołowicz, Krzysztof Kazimierczuk, Mariusz Pietrzak, Sławomir Szymański
Triptycene derivatives are widely utilized in different fields of chemistry and materials sciences. Their physicochemical properties, often of pivotal importance for the rational design of triptycene‐based functional materials, are influenced by noncovalent interactions between substituents mounted on the triptycene skeleton. Here, a unique interaction between electron‐rich substituents in the peri position and the silyl group located on the bridgehead sp3‐carbon is discussed on the example of 1,4‐dichloro‐9‐(p‐methoxyphenyl)‐silyltriptycene (TRPCl) which exists in solution in the form of two rotamers differing by dispositions, syn or anti, of the Si‐CPh (the CPh atom is from the p‐methoxyphenyl group) bond against the peri‐Cl atom. For the first time, substantial differences between the Si‐CPh bonds in these two dispositions are identified, based on indirect experimental and direct theoretical evidence. For these two orientations, the experimental 1J(Si,CPh) values differ by as much as 10 percent. The differences are explained in terms of effective electron density transfers from the peri‐Cl atom to the antibonding s* orbitals of the Si‐X bonds (X=H, CPh) oriented anti to that atom. The electronic effects are revealed by an NBO analysis. Connections of these observations with the notion of blue‐shifting hydrogen bonds are discussed.
New Article in Analytical Chemistry
08 August 2019Non-stationary 2D NMR – a novel method for studying reaction mechanism in situ.
Ewa K. Nawrocka, Paweł Kasprzak, Katarzyna Zawada, Jarosław Sadło, Wojciech Grochala, Krzysztof Kazimierczuk* and Piotr J. Leszczyński*
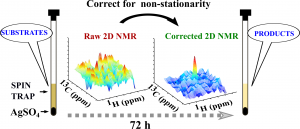
Nuclear magnetic resonance spectroscopy (NMR) is a versatile tool of chemical analysis allowing to determine structures of molecules with atomic resolution. Particularly informative are two-dimensional (2D) experiments, that directly identify atoms coupled by chemical bonds or a through-space interaction. Thus, NMR could potentially be powerful tool to study reactions in situ and explain their mechanisms. Unfortunately, 2D NMR is very time-consuming and thus often cannot serve as a ”snapshot” technique for in situ reaction monitoring. Particularly difficult is the case of spectra, in which resonance frequencies vary in the course of reaction. This leads to resolution and sensitivity loss, often hindering the detection of transient products. In this paper we introduce a novel approach to correct such non-stationary 2D NMR signals and raise the detection limits over 10 times. We demonstrate success of its application for studying the mechanism of the reaction of AgSO4-induced synthesis of diphenylmethane-type compounds. Several reactions occur in the studied mixture of benzene and toluene, all with rather low yield and leading to compounds with similar chemical shifts.Nevertheless, with the use of a proposed 2D NMR approach we were able to describe complex mechanisms of diphenylmethane formation involving AgSO4-induced toluene deprotonation and formation of benzyl carbocation, followed by nucleophylic attacks.
New Article in Physical Chemistry Chemical Physics
Quick temperature-swept pure-shift NMR: case of solvent effects in atorvastatin
Małgorzata Rytel, Paweł Kasprzak, Piotr Setny and Krzysztof Kazimierczuk
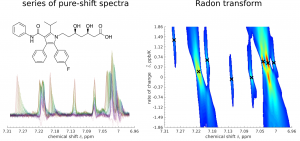
Pure-shift NMR experiments provide highly resolved spectra, that could be perfect for precise monitoring of chemical shift variations under different conditions, such as temperature or concentration. However, their sensitivity is quite low and signal sampling is time-consuming, leading to long experimental times and making such serial acquisition problematic. In this paper we present a new method of NMR spectroscopy improving the speed and sensitivity of serial pseudo-two-dimensional pure-shift experiments. The example of variable-temperature study of atorvastatin reveals the potential of the method in verifying the theoretical predictions of solvent-dependent spectral effects.
New Article in Chemical Communications
Accelerated acquisition in pure-shift spectra based on prior knowledge from 1H NMR
Alexandra Shchukina, Magdalena Kaźmierczak, Paweł Kasprzak, Matthew Davy, Geoffrey R. Akien, Craig P. Butts and Krzysztof Kazimierczuk
Pure-shift NMR enhances spectral resolution, but the optimal resolution can only be obtained at the cost of acquisition time. We propose to accelerate pure-shift acquisition using optimised ‘burst’ non-uniform sampling schemes [I. E. Ndukwe, A. Shchukina, K. Kazimierczuk and C. P. Butts, Chem. Commun., 2016, 52, 12769] and then reconstruct the undersampled signal mathematically. Here, we focus on the reliability of this reconstruction depending on the sampling scheme and present a workflow for the sampling optimization. It is ready to be implemented in routine measurements and yields a great improvement in reconstruction of challenging cases.
New Article in ChemistryOpen
12 March 2019Monitoring Hydrogenation Reactions using Benchtop 2D NMR with Extraordinary Sensitivity and Spectral Resolution
Dariusz Gołowicz, Krzysztof Kazimierczuk, Mateusz Urbańczyk, Tomasz Ratajczyk

Low‐field benchtop nuclear magnetic resonance (BT‐NMR) spectrometers with Halbach magnets are being increasingly used in science and industry as cost‐efficient tools for the monitoring of chemical reactions, including hydrogenation. However, their use of low‐field magnets limits both resolution and sensitivity. In this paper, we show that it is possible to alleviate these two problems through the combination of parahydrogen‐induced polarization (PHIP) and fast correlation spectroscopy with time‐resolved non‐uniform sampling (TR‐NUS). PHIP can enhance NMR signals so that substrates are easily detectable on BT‐NMR spectrometers. The interleaved acquisition of one‐ and two‐dimensional spectra with TR‐NUS provides unique insight into the consecutive moments of hydrogenation reactions, with a spectral resolution unachievable in a standard approach. We illustrate the potential of the technique with two examples: the hydrogenation of ethylphenyl propiolate and the hydrogenation of a mixture of two substrates – ethylphenyl propiolate and ethyl 2‐butynoate.