New Article in Analyst
07 September 2020Enhancing benchtop NMR spectroscopy by means of sample shifting
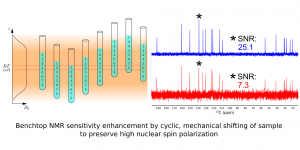
Benchtop NMR spectrometers have become widely available over the last decade. They are now used successfully in various branches of chemistry. Their popularity continues to grow due to their low price and almost zero running costs. However, benchtop spectrometers perform much less effectively than the high-field spectrometers used in NMR labs for several decades. In this article we present a solution for boosting the sensitivity of benchtop NMR spectrometers in a multi-scan experiment and improving its capabilities in quantitative measurement. Our solution involves the synchronized shifting of a sample to preserve its high nuclear polarization during the measurement. We performed several experiments using different samples to confirm this improved performance: an1H NMR experiment for 4,4-Dimethoxy-2-butanone, and13C NMR experiments for benzyl salicylate,liquid pharmaceutical product Acerin (skin solution), and a mixture of m-Anisaldehyde and (R)-(+)-Limonene.
New Article in J. of Biomolecular NMR
A novel high-dimensional NMR experiment for resolving protein backbone dihedral angle ambiguities
Clemens Kauffmann, Krzysztof Kazimierczuk, Thomas C. Schwarz, Robert Konrat & Anna Zawadzka-Kazimierczuk
Intrinsically disordered proteins (IDPs) are challenging established structural biology perception and urge a reassessment of the conventional understanding of the subtle interplay between protein structure and dynamics. Due to their importance in eukaryotic life and central role in protein interaction networks, IDP research is a fascinating and highly relevant research area in which NMR spectroscopy is destined to be a key player. The flexible nature of IDPs, as a result of the sampling of a vast conformational space, however, poses a tremendous scientific challenge, both technically and theoretically. Pronounced signal averaging results in narrow signal dispersion and requires higher dimensionality NMR techniques. Moreover, a fundamental problem in the structural characterization of IDPs is the definition of the conformational ensemble sampled by the polypeptide chain in solution, where often the interpretation relies on the concept of ‘residual structure’ or ‘conformational preference’. An important source of structural information is information-rich NMR experiments that probe protein backbone dihedral angles in a unique manner. Cross-correlated relaxation experiments have proven to fulfil this task as they provide unique information about protein backbones, particularly in IDPs. Here we present a novel cross-correlation experiment that utilizes non-uniform sampling detection schemes to resolve protein backbone dihedral ambiguities in IDPs. The sensitivity of this novel technique is illustrated with an application to the prototypical IDP 𝛼α-Synculein for which unexpected deviations from random-coil-like behaviour could be observed.
New Article in PLOS
Restriction of S-adenosylmethionine conformational freedom by knotted protein binding sites
Agata P. Perlinska, Adam Stasiulewicz, Ewa K. Nawrocka, Krzysztof Kazimierczuk, Piotr Setny, Joanna I. Sulkowska
S-adenosylmethionine (SAM) is one of the most important enzyme substrates. It is vital for the function of various proteins, including large group of methyltransferases (MTs). Intriguingly, some bacterial and eukaryotic MTs, while catalysing the same reaction, possess significantly different topologies, with the former being a knotted one. Here, we conducted a comprehensive analysis of SAM conformational space and factors that affect its vastness. We investigated SAM in two forms: free in water (via NMR studies and explicit solvent simulations) and bound to proteins (based on all data available in the PDB and on all-atom molecular dynamics simulations in water). We identified structural descriptors—angles which show the major differences in SAM conformation between unknotted and knotted methyltransferases. Moreover, we report that this is caused mainly by a characteristic for knotted MTs compact binding site formed by the knot and the presence of adenine-binding loop. Additionally, we elucidate conformational restrictions imposed on SAM molecules by other protein groups in comparison to conformational space in water.
New Article in Sensors
02 March 2020Enhancing Compression Level for More Efficient Compressed Sensing and Other Lessons from NMR Spectroscopy
Dariusz Gołowicz, Paweł Kasprzak and Krzysztof Kazimierczuk*
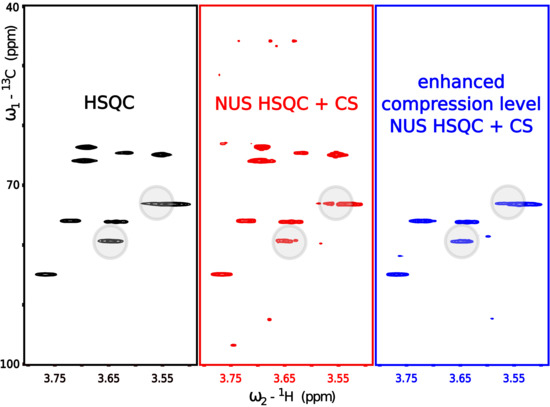
Modern nuclear magnetic resonance spectroscopy (NMR) is based on two- and higher-dimensional experiments that allow the solving of molecular structures, i.e., determine the relative positions of single atoms very precisely. However, rich chemical information comes at the price of long data acquisition times (up to several days). This problem can be alleviated by compressed sensing (CS)—a method that revolutionized many fields of technology. It is known that CS performs the most efficiently when measured objects feature a high level of compressibility, which in the case of NMR signal means that its frequency domain representation (spectrum) has a low number of significant points. However, many NMR spectroscopists are not aware of the fact that various well-known signal acquisition procedures enhance compressibility and thus should be used prior to CS reconstruction. In this study, we discuss such procedures and show to what extent they are complementary to CS approaches. We believe that the survey will be useful not only for NMR spectroscopists but also to inspire the broader signal processing community.
New Article in ChemPhysChem
23 January 2020Blue‐shift hydrogen bonds in silyltriptycene derivatives. Antibonding σ* orbitals of Si‐C bond as effective acceptors of electron density
Tomasz Ratajczyk*, Adam Mames, Dariusz Gołowicz, Krzysztof Kazimierczuk, Mariusz Pietrzak, Sławomir Szymański
Triptycene derivatives are widely utilized in different fields of chemistry and materials sciences. Their physicochemical properties, often of pivotal importance for the rational design of triptycene‐based functional materials, are influenced by noncovalent interactions between substituents mounted on the triptycene skeleton. Here, a unique interaction between electron‐rich substituents in the peri position and the silyl group located on the bridgehead sp3‐carbon is discussed on the example of 1,4‐dichloro‐9‐(p‐methoxyphenyl)‐silyltriptycene (TRPCl) which exists in solution in the form of two rotamers differing by dispositions, syn or anti, of the Si‐CPh (the CPh atom is from the p‐methoxyphenyl group) bond against the peri‐Cl atom. For the first time, substantial differences between the Si‐CPh bonds in these two dispositions are identified, based on indirect experimental and direct theoretical evidence. For these two orientations, the experimental 1J(Si,CPh) values differ by as much as 10 percent. The differences are explained in terms of effective electron density transfers from the peri‐Cl atom to the antibonding s* orbitals of the Si‐X bonds (X=H, CPh) oriented anti to that atom. The electronic effects are revealed by an NBO analysis. Connections of these observations with the notion of blue‐shifting hydrogen bonds are discussed.
New Article in Progress in NMR Spectroscopy
30 September 2019Fast time-resolved NMR with non-uniform sampling

Dariusz Gołowicz, Paweł Kasprzak, Vladislav Orekhov and Krzysztof Kazimierczuk*
NMR spectroscopy is a versatile tool for studying time-dependent processes: chemical reactions, phase transitions or macromolecular structure changes. However, time-resolved NMR is usually based on the simplest among available techniques – one-dimensional spectra serving as “snapshots” of the studied process. One of the reasons is that multidimensional experiments are very time-expensive due to costly sampling of evolution time space. In this review we summarize efforts to alleviate the problem of limited applicability of multidimensional NMR in time-resolved studies. We focus on techniques based on sparse or non- uniform sampling (NUS), which lead to experimental time reduction by omitting a significant part of the data during measurement and reconstructing it mathematically, adopting certain assumptions about the spectrum. NUS spectra are faster to acquire than conventional ones and thus better suited to the role of “snapshots”, but still suffer from non-stationarity of the signal i.e. amplitude and frequency variations within a dataset. We discuss in detail how these instabilities affect the spectra, and what are the optimal ways of sampling the non-stationary FID signal. Finally, we discuss related areas of NMR where serial experiments are exploited and how they can benefit from the same NUS-based approaches.

