New Article in ChemPhysChem
25 January 2021Blue‐Shift Hydrogen Bonds in Silyltriptycene Derivatives: Antibonding σ* Orbitals of the Si−C Bond as Effective Acceptors of Electron Density
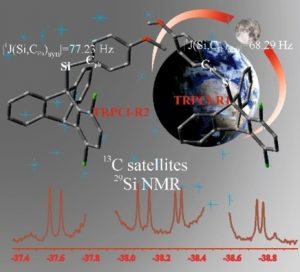
Dr. Adam Mames, Dariusz Gołowicz, Dr. Mariusz Pietrzak, Dr. Krzysztof Kazimierczuk, Prof. Sławomir Szymański, Dr. Tomasz Ratajczyk
Triptycene derivatives are widely utilized in different fields of chemistry and materials sciences. Their physicochemical properties, often of pivotal importance for the rational design of triptycene‐based functional materials, are influenced by noncovalent interactions between substituents mounted on the triptycene skeleton. Herein, a unique interaction between electron‐rich substituents in the peri position and the silyl group located on the bridgehead sp3‐carbon is discussed on the example of 1,4‐dichloro‐9‐(p‐methoxyphenyl)‐silyltriptycene (TRPCl) which exists in solution in the form of two rotamers differing by dispositions, syn or anti, of the Si−CPh (the CPh atom is from the p‐methoxyphenyl group) bond against the peri‐Cl atom. For the first time, substantial differences between the Si−CPh bonds in these two dispositions are identified, based on indirect experimental and direct theoretical evidence. For these two orientations, the experimental 1J(Si,CPh) values differ by as much as 10 percent. The differences are explained in terms of effective electron density transfer from the peri‐Cl atom to the antibonding σ* orbitals of the Si−X bonds (X=H, CPh) oriented anti to that atom. The electronic effects are revealed by an NBO analysis. Connections of these observations with the notion of blue‐shifting hydrogen bonds are discussed.
New Article in RSC Advances
Toward the synthesis, fluorination and application of N–graphyne
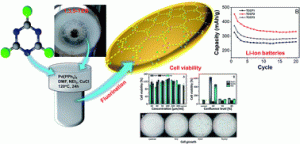
Gisya Abdi, Anna Filip, Michał Krajewski, Krzysztof Kazimierczuk, Marcin Strawski, Paweł Szarek, Bartosz Hamankiewicz, Zoran Mazej, Grzegorz Cichowicz Piotr J. Leszczyński, Karol J. Fijałkowski and Andrzej Szczurek
The discovery of properties and applications of unknown materials is one of the hottest research areas in materials science. In this work, we navigate a route towards these goals by the development of a new type of graphyne nanostructure. It is synthesised by a Sonogashira cross-coupling reaction of 1,3,5-triethynylbenzene with cyanuric chloride resulting in an extended carbon-based material called TCC. Also, we modify the obtained TCC via fluorination using XeF2 at various concentrations to investigate the effect of fluorination on the triple bonds and the conjugated structure of graphyne. In this study, we put special emphasis on the determination of the impact of the fluorine content and the type of CF functionalities on the morphology, chemical and electronic structure, biocompatibility, electrical conductivity and possible applicability as anode materials for Li-ion batteries. The obtained results indicate that the character of C–F bonds influences the final properties of fluorinated materials. The polar C–F bonds are preferable for cell proliferation while CF2 groups are most suitable for battery devices, however, the appearance of PTFE-like units may have a negative impact on battery specific capacitance as well as on cell viability.
New article in Bulletin of the Russian Academy of Sciences: Physics
On Jackknifed Greedy Algorithms and Their Applications in NMR
Paweł Kasprzak, Krzysztof Kazimierczuk, Alexanda Shchukina *
Multidimensional NMR experiments applied in e.g. protein NMR may take days or even weeks of expensive experimental time. Compressed sensing (CS) is a method to save this time: some measurements are skipped and then reconstructed with the help of special algorithms. The orthogonal matching pursuit (OMP) is one of the effective tools of compressed sensing field. In this paper we introduce a jackknife-type stopping criterion for OMP. Unlike the standard stopping criteria, it does not require an estimation of the noise level. We extend the concept to the lorentzian peaks matching pursuit (LPMP) algorithm.
New Article in Magnetic Resonance in Chemistry
09 November 2020Benefits of time‐resolved nonuniform sampling in reaction monitoring: The case of aza‐Michael addition of benzylamine and acrylamide

Dariusz Gołowicz, Magdalena Kaźmierczak and Krzysztof Kazimierczuk*
Time‐resolved nonuniform sampling (TR‐NUS) applied to reaction monitoring eliminates 2D spectral line disturbances resulting from varying peak intensities and positions. This allows, in turn, avoiding wrong conclusions about kinetics. These considerations are exemplified with a seemingly simple aza‐Michael reaction of benzylamine and acrylamide. Surprisingly, the product identification is possible only using 2D spectra, although credible monitoring requires TR‐NUS.
New Article in Angewandte Chemie
07 September 2020Non‐stationary complementary non‐uniform sampling (NOSCO NUS) for fast acquisition of serial 2D NMR titration data
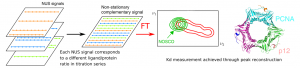
NMR spectroscopy offers unique benefits for ligand binding studies on isotopically labelled target proteins, such as atomic resolution, direct distinction of binding sites and modes, lowest detectable affinity limit, and function independent setup. Yet, retracing protein signal assignments from apo to holo states to derive exact dissociation constants and Chemical Shift Perturbation amplitudes (for ligand docking and structure‐based optimization) requires lengthy titration series of 2D heteronuclear correlation spectra at variable ligand concentration that may exceed the protein’s lifetime and available spectrometer time. We present a novel method to overcome this critical limitation, based on non‐stationary complementary non‐uniform sampling (NOSCO NUS) combined with a robust particle swarm algorithm. We illustrate its potential in two challenging studies with very distinct protein size and binding affinities, showing that NOSCO NUS can reduce measurement times by an order of magnitude to make such highly informative NMR titration studies more broadly feasible.
New Article in Analyst
Enhancing benchtop NMR spectroscopy by means of sample shifting
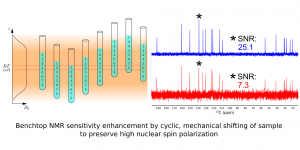
Benchtop NMR spectrometers have become widely available over the last decade. They are now used successfully in various branches of chemistry. Their popularity continues to grow due to their low price and almost zero running costs. However, benchtop spectrometers perform much less effectively than the high-field spectrometers used in NMR labs for several decades. In this article we present a solution for boosting the sensitivity of benchtop NMR spectrometers in a multi-scan experiment and improving its capabilities in quantitative measurement. Our solution involves the synchronized shifting of a sample to preserve its high nuclear polarization during the measurement. We performed several experiments using different samples to confirm this improved performance: an1H NMR experiment for 4,4-Dimethoxy-2-butanone, and13C NMR experiments for benzyl salicylate,liquid pharmaceutical product Acerin (skin solution), and a mixture of m-Anisaldehyde and (R)-(+)-Limonene.

