Non‐stationary complementary non‐uniform sampling (NOSCO NUS) for fast acquisition of serial 2D NMR titration data
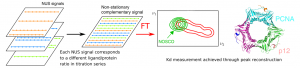
NMR spectroscopy offers unique benefits for ligand binding studies on isotopically labelled target proteins, such as atomic resolution, direct distinction of binding sites and modes, lowest detectable affinity limit, and function independent setup. Yet, retracing protein signal assignments from apo to holo states to derive exact dissociation constants and Chemical Shift Perturbation amplitudes (for ligand docking and structure‐based optimization) requires lengthy titration series of 2D heteronuclear correlation spectra at variable ligand concentration that may exceed the protein’s lifetime and available spectrometer time. We present a novel method to overcome this critical limitation, based on non‐stationary complementary non‐uniform sampling (NOSCO NUS) combined with a robust particle swarm algorithm. We illustrate its potential in two challenging studies with very distinct protein size and binding affinities, showing that NOSCO NUS can reduce measurement times by an order of magnitude to make such highly informative NMR titration studies more broadly feasible.

